Consider the following reversible reaction.
what is the equilibrium constant expression...

Chemistry, 24.11.2019 08:31, vapelordcarl69
Consider the following reversible reaction.
what is the equilibrium constant expression for the given system?
1st pic is equation, 2nd is a, 3rd is b, 4th is c, and 5th is d.
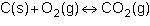


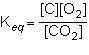


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Do you know the correct answer?
Questions in other subjects:






Biology, 01.08.2019 17:00

Mathematics, 01.08.2019 17:00


Biology, 01.08.2019 17:00

History, 01.08.2019 17:00






