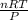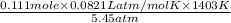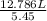Chemistry, 17.12.2019 04:31, khikhi1705
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperature of 1130 degrees celsius at a pressure of 5.45 atmospheres? (the ideal gas constant is 0.0821 l•atm/mol•k.)
0.43 liters
1.43 liters
1.89 liters
2.35 liters

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperat...
Questions in other subjects:


Computers and Technology, 13.12.2021 17:10

Mathematics, 13.12.2021 17:10


Computers and Technology, 13.12.2021 17:10


English, 13.12.2021 17:10


Mathematics, 13.12.2021 17:10

Biology, 13.12.2021 17:10