
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases from 25.20 °c to 27.60 °c. the calorimeter contains 1.17×103 g of water and the bomb has a heat capacity of 786 j/°c. based on this experiment, calculate δe for the combustion reaction per mole of azulene burned (kj/mol). c13h24o4(s) + 17 o2(g) 13 co2(g) + 12 h2o(l) e = kj/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, brittanygibson2812
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 09:00, pinapunapula
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
Do you know the correct answer?
A0.327-g sample of azulene (c10h8) is burned in a bomb calorimeter and the temperature increases fro...
Questions in other subjects:

English, 23.03.2021 23:40




Computers and Technology, 23.03.2021 23:40


Mathematics, 23.03.2021 23:40

Chemistry, 23.03.2021 23:40

History, 23.03.2021 23:40


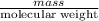
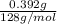
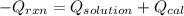
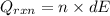
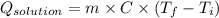
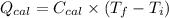
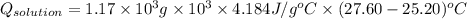
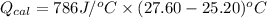
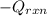 = (11748.67 + 1886.4) J
= (11748.67 + 1886.4) J
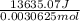
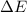 for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.
for the given combustion reaction per mole of azulene burned is 4452.26 kJ/mol.



