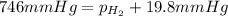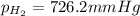Chemistry, 23.11.2019 05:31, Simplytaylorgrenade
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement in a 22 °c water bath. the barometric pressure in the lab that day was 746 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
Do you know the correct answer?
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. t...
Questions in other subjects:

Geography, 22.09.2019 02:10

Chemistry, 22.09.2019 02:10

Mathematics, 22.09.2019 02:10

English, 22.09.2019 02:10


Biology, 22.09.2019 02:10



Arts, 22.09.2019 02:10


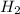 is, 726.2 mmHg
is, 726.2 mmHg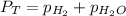
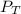 = total partial pressure = barometric pressure = 746 mmHg
= total partial pressure = barometric pressure = 746 mmHg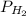 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?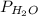 = partial pressure of water vapor = 19.8 mmHg (assume)
= partial pressure of water vapor = 19.8 mmHg (assume)