
Chemistry, 23.11.2019 04:31, Jenifermorales101
It turns out that the van der waals constant b equals four times the total volume actually occupied by the molecules of a mole of gas.
using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms at 230 atm pressure and 0 c. assume b=0.0322 l/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3


Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
Do you know the correct answer?
It turns out that the van der waals constant b equals four times the total volume actually occupied...
Questions in other subjects:

Geography, 19.11.2020 17:00

Mathematics, 19.11.2020 17:00

Geography, 19.11.2020 17:00

Mathematics, 19.11.2020 17:00




English, 19.11.2020 17:00


Mathematics, 19.11.2020 17:00


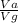
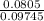 = 0.0826
= 0.0826



