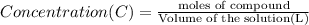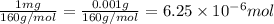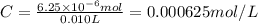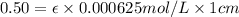Chemistry, 23.11.2019 00:31, chelseychew32
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the solution is poured into a 1-cm uv cell. the uv spectrum is taken, and there is an absorption at = 247 nm. the maximum absorbance at 247 nm is 0.50. calculate the value of for this absorption.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, kaitlyn2030
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
Do you know the correct answer?
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the soluti...
Questions in other subjects:


Mathematics, 30.12.2019 18:31



Biology, 30.12.2019 18:31



Social Studies, 30.12.2019 18:31

History, 30.12.2019 18:31

Mathematics, 30.12.2019 18:31


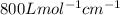 .
.
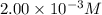
 = molar absorptivity coefficient
= molar absorptivity coefficient