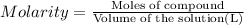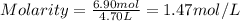Chemistry, 22.11.2019 23:31, shealwaysknows23
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient water so that the final volume of the solution is 4.70 l . calculate the molarity of the mgcl 2 solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1
Do you know the correct answer?
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient wate...
Questions in other subjects:




Mathematics, 01.03.2021 20:00


English, 01.03.2021 20:00

Mathematics, 01.03.2021 20:00

English, 01.03.2021 20:00