
Chemistry, 22.11.2019 22:31, maheshwarlall
The value of δg° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2so3 (g) → 2s (s, rhombic) + 3o2 (g) is kj/mol. the value of g° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2so3 (g) 2s (s, rhombic) + 3o2 (g) is kj/mol. +740.8 -370.4 +185.2 +370.4 -740.8

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
Do you know the correct answer?
The value of δg° at 25 °c for the decomposition of gaseous sulfur trioxide to solid elemental sulfur...
Questions in other subjects:








Social Studies, 09.12.2021 02:30


Mathematics, 09.12.2021 02:30




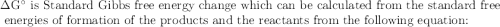
![\begin{array}{l}{\Delta \mathrm{G}^{\circ}=\Sigma \mathrm{G}_{\mathrm{f}(\text { products })}^{\circ}-\Sigma \mathrm{G}_{\text {creatants }}^{\circ}} \\ {\Delta \mathrm{G}^{\circ}=[\mathrm{Sum} \text { of standard free energies of formation of products }]-[\mathrm{Sum}\text { of standard } } \\ {\text { free energies bf formation of reactants] }}\end{array}](/tpl/images/0386/9118/6152f.png)
![\begin{array}{l}{\text { Now here standard values of } \Delta G^{\circ} f(k J / m o l) \text { for } S=0, O_{2}=0 \& S O_{3}=-370.4} \\ {\text { Hence these values can be substituted in above equation: }} \\ {\Delta G^{\circ}=\left[2 G_{f}^{\circ}(0)+3 G_{f}(0)\right]-[2(-370.4)]} \\ {\Delta G^{\circ}=[-0+0]-[-740.8]} \\ {\Delta G^{\circ}=+740.8 \mathrm{kJ} / \mathrm{mol}}\end{array}](/tpl/images/0386/9118/e4e39.png)




