
Chemistry, 22.11.2019 21:31, samueltaye
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0°c, are placed in contact in an isolated container. the specific heat capacity of copper is 0.385 j k−1 g−1 and may be assumed constant over the temperature range involved.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 10:30, staceymrtosr
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2
Do you know the correct answer?
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0...
Questions in other subjects:


Social Studies, 24.08.2019 19:10

Mathematics, 24.08.2019 19:10

Geography, 24.08.2019 19:10







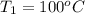 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K 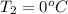 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K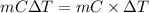
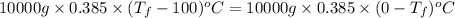
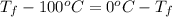
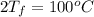
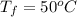
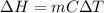
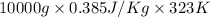
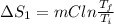
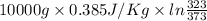
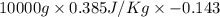
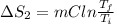
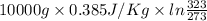
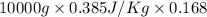
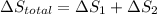
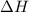 is 1243.5 kJ and
is 1243.5 kJ and 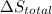 is 93.37 J/K.
is 93.37 J/K.



