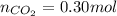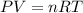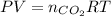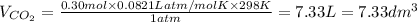Chemistry, 04.11.2019 14:31, danielzgame
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.10 mol of propan-1-ol. what is the final volume of gas, measured under room conditions? a 7.20 dm3 b 8.40 dm3 c 16.8 dm3 d 18.00 dm3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Do you know the correct answer?
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.1...
Questions in other subjects:

Mathematics, 02.07.2020 23:01

Health, 02.07.2020 23:01




Mathematics, 02.07.2020 23:01



Mathematics, 02.07.2020 23:01


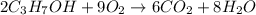
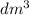 )
)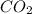 then, 0.10 moles of propanol will give:
then, 0.10 moles of propanol will give: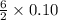 moles of
moles of