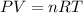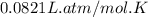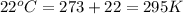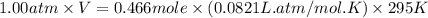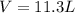Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated, thus inflating the bag. 2nan3(s) > 2na(s) + 3n2(g) calculate the value of w (work) for the following system if 20.2 g of nan3 reacts completely at 1.00 atm and 22 degrees

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
Do you know the correct answer?
Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated...
Questions in other subjects:

Mathematics, 22.09.2019 09:30


English, 22.09.2019 09:30



Mathematics, 22.09.2019 09:30


History, 22.09.2019 09:30

Social Studies, 22.09.2019 09:30

Chemistry, 22.09.2019 09:30


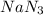
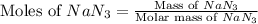
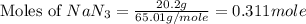
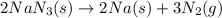
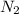
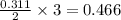 moles of
moles of