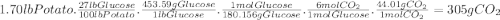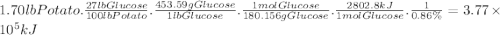Chemistry, 22.11.2019 04:31, mclendenen8011
Photosynthesis allows plants to turn light energy into chemical energy by forming glucose from carbon dioxide and water: 6co2(g) + 6h2o(l) → c6h12o6(g) + o2(g) δhrxn = 2802.8 kj the glucose is then used to form complex carbohydrates that the plant needs to survive. a 1.70 lb sweet potato is approximately 73% water by mass. if the remaining mass is made up of carbohydrates derived from glucose (mw = 180.156 g/mol), how much carbon dioxide (mw = 44.01 g/mol) was needed to grow this sweet potato? g how much light energy does it take to grow the 1.70 lb. sweet potato if the efficiency of photosynthesis is 0.86%? kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
Photosynthesis allows plants to turn light energy into chemical energy by forming glucose from carbo...
Questions in other subjects:

Mathematics, 25.07.2019 15:30

English, 25.07.2019 15:30

English, 25.07.2019 15:30

Mathematics, 25.07.2019 15:30

Social Studies, 25.07.2019 15:30

Chemistry, 25.07.2019 15:30

History, 25.07.2019 15:30

Social Studies, 25.07.2019 15:30

History, 25.07.2019 15:30