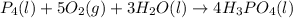Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: p4(l)+5o2(g-2 p20s(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acld p20(9)+3 h200 2h, po40) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3


Do you know the correct answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions in other subjects:

Mathematics, 10.12.2020 04:40


Mathematics, 10.12.2020 04:40


Mathematics, 10.12.2020 04:40

History, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40



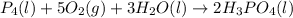
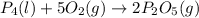 ......(1)
......(1)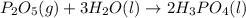 .......(2)
.......(2)
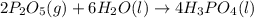 .......(3)
.......(3)