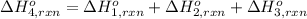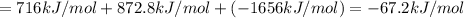Chemistry, 22.11.2019 03:31, CyberSongWriter
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estimate the standard enthalpy of formation of methane (ch4). c(s) → c(g) δh o rxn = 716 kj/mol 2h2(g) → 4h(g) δh o rxn = 872.8 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, alexisgoss8091
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
Do you know the correct answer?
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estim...
Questions in other subjects:




Arts, 27.02.2020 22:50

History, 27.02.2020 22:50

Mathematics, 27.02.2020 22:50





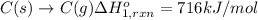 ...[1]
...[1]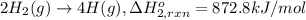 ...[2]
...[2]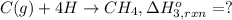 ...[3]
...[3]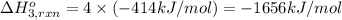
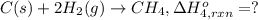 ...[4]
...[4]