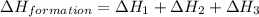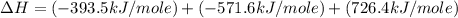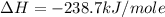Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 --> co2(g) latex: \deltaδh° = –393.5 kj/mol h2(g) + (1/2)o2 --> h2o(l) latex: \deltaδh° = –285.8 kj/mol ch3oh(l) + (3/2)o2(g) --> co2(g) + 2h2o(l) latex: \deltaδh° = –726.4 kj/mol

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 16:30, monnicawilliam
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
Do you know the correct answer?
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following infor...
Questions in other subjects:



Social Studies, 04.04.2020 00:37

History, 04.04.2020 00:38







 will be,
will be,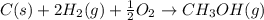
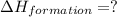
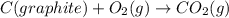
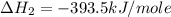
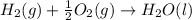
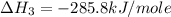
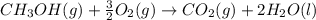
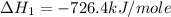
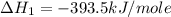
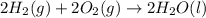
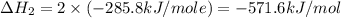
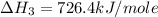
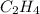 will be,
will be,