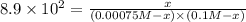Chemistry, 22.11.2019 01:31, ellenaschool
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through the formation of the red fe(h2o)5scn2+ (or, more simply, fescn2+). what is [fe3+] when 0.700 l each of 0.00150 m fe(no3)3 and 0.200 m kscn are mixed? kf of fescn2+ = 8.9 × 102. enter your answer in scientific notation. report your final answer to two significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through t...
Questions in other subjects:

Mathematics, 30.01.2021 15:40



English, 30.01.2021 15:40


Physics, 30.01.2021 15:40

Mathematics, 30.01.2021 15:40

Social Studies, 30.01.2021 15:40

English, 30.01.2021 15:40


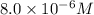 .
.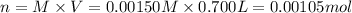
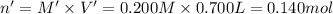
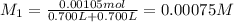
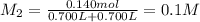
![Fe^{3+}+SCN^-\rightleftharpoons [Fe(SCN)]^{2+}](/tpl/images/0385/5120/942bc.png)
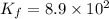
![K_f=\frac{[[Fe(SCN)]^{2+}]}{[[Fe^{3+}]][SCN^{-}]}](/tpl/images/0385/5120/f34ae.png)