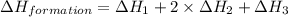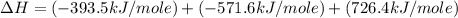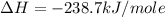Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c(graphite) + o2(g) co2(g) δh°rxn = -393.5 kj h2(g) + 1/2 o2(g) h2o(l) δh°rxn = -285.8 kj calculate the enthalpy of formation of methanol (ch3oh) from its elements.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
Do you know the correct answer?
Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c...
Questions in other subjects:

History, 18.08.2019 10:50

Mathematics, 18.08.2019 10:50

Mathematics, 18.08.2019 10:50



Mathematics, 18.08.2019 10:50

Physics, 18.08.2019 10:50




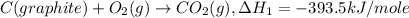 ..[1]
..[1]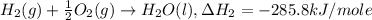 ..[2]
..[2]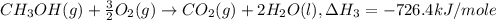 ..[3]
..[3]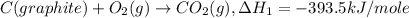 ..[1]
..[1]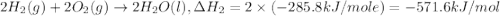 ..[2]
..[2]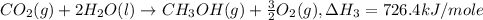 [3]
[3]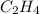 will be,
will be,