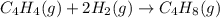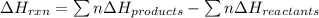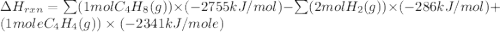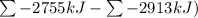Chemistry, 22.11.2019 00:31, montrellgoodman5890
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. using the enthalpies of combustion for c4h4 (-2341 kj/mol), c4h8 (-2755 kj/mol), and h2 (-286 kj/mol), calculate δh for the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
Do you know the correct answer?
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and h...
Questions in other subjects:


Mathematics, 31.03.2020 01:33





Mathematics, 31.03.2020 01:33