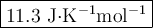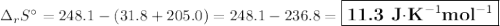The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) → so2 (g) is j/k⋅mol. the value of s° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombic) + o2 (g) so2 (g) is j/kmol. +248.5 +485.4 +11.6 -11.6 -248.5

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Do you know the correct answer?
The value of δs° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, s (s, rhombi...
Questions in other subjects:


Mathematics, 09.11.2020 22:00

Biology, 09.11.2020 22:00


English, 09.11.2020 22:00

Mathematics, 09.11.2020 22:00

Mathematics, 09.11.2020 22:00


Mathematics, 09.11.2020 22:00