
Chemistry, 21.11.2019 23:31, katielloyd
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c. if the temperature of the calorimeter increased from 22.36 °c to 28.78 °c, then what is the δh for the combustion of one mole of octane? do not add the unit in the answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:00, rileyallen4186pd5tgy
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Do you know the correct answer?
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c....
Questions in other subjects:

Social Studies, 23.04.2021 16:10


Mathematics, 23.04.2021 16:10


Geography, 23.04.2021 16:10



Physics, 23.04.2021 16:10

Mathematics, 23.04.2021 16:10


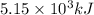
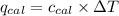
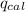 = Heat gained by bomb calorimeter
= Heat gained by bomb calorimeter 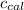 =Heat capacity of bomb calorimeter=12.66 kJ/°C
=Heat capacity of bomb calorimeter=12.66 kJ/°C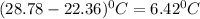
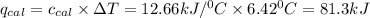
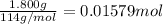
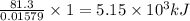 of heat
of heat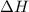 for the combustion of one mole of octane is
for the combustion of one mole of octane is 



