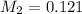Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
Do you know the correct answer?
If 0.290g of fas is dissolved in 10ml of di water and is titrated to equivalence with 12.23ml kmno4,...
Questions in other subjects:




Mathematics, 08.02.2021 21:00


Mathematics, 08.02.2021 21:00

Mathematics, 08.02.2021 21:00



Mathematics, 08.02.2021 21:00


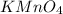 is 0.121 M
is 0.121 M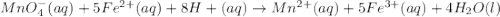
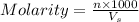
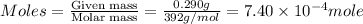
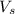 = volume of solution = 10 ml
= volume of solution = 10 ml
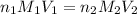
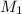 = molarity of
= molarity of 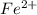 solution = 0.074 M
solution = 0.074 M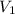 = volume of
= volume of 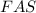 solution = 10 ml
solution = 10 ml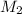 = molarity of
= molarity of 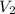 = volume of
= volume of 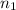 = valency of
= valency of 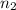 = valency of
= valency of