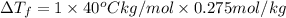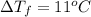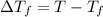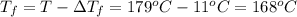Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
Do you know the correct answer?
Assume the molality of isoborneol in your product is 0.275 mol/kg. what is the melting point of your...
Questions in other subjects:


Mathematics, 02.03.2021 16:40


Mathematics, 02.03.2021 16:40


Mathematics, 02.03.2021 16:40

Mathematics, 02.03.2021 16:40


SAT, 02.03.2021 16:40


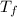 = ?
= ?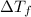
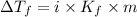
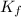 = The freezing point depression constant
= The freezing point depression constant