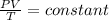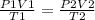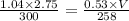Chemistry, 21.11.2019 22:31, darnellgee298
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reaches an altitude where the temperature is -15 °c and the pressure is 0.530 atm. calculate the final volume of the balloon

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mimithurmond03
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1


Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reac...
Questions in other subjects:





Mathematics, 22.06.2019 11:40

Biology, 22.06.2019 11:40

Chemistry, 22.06.2019 11:40

Mathematics, 22.06.2019 11:40

Chemistry, 22.06.2019 11:40

History, 22.06.2019 11:40