
Two nonpolar organic liquids, hexane (c_6h_14) and heptane (c_7h_16) are mixed. hexane and heptane are miscible with each other in all proportions. in making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure liquids?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
Do you know the correct answer?
Two nonpolar organic liquids, hexane (c_6h_14) and heptane (c_7h_16) are mixed. hexane and heptane a...
Questions in other subjects:


Mathematics, 30.01.2022 01:00


Mathematics, 30.01.2022 01:00







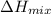 is nearly zero)
is nearly zero)



