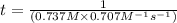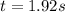Chemistry, 21.11.2019 21:31, treytonmesser
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m-1 s-1. if the initial concentration of the reaction was 0.737 m, what is the half life for the reaction?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
Do you know the correct answer?
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m...
Questions in other subjects:

History, 08.12.2019 06:31


Mathematics, 08.12.2019 06:31

Mathematics, 08.12.2019 06:31







![\frac{1}{[A]_{t}}=kt+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/16aaf.png)
![[A]_{t}](/tpl/images/0385/0648/c37dd.png) is concentration of A after "t" time and
is concentration of A after "t" time and ![[A]_{0}](/tpl/images/0385/0648/48818.png) is initial concentration of A
is initial concentration of A![[A]_{t}=\frac{[A]_{0}}{2}](/tpl/images/0385/0648/2b76d.png)
![[A]_{0}=0.737M](/tpl/images/0385/0648/c3ec8.png) and
and 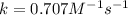
![\frac{1}{\frac{[A]_{0}}{2}}=(0.707M^{-1}s^{-1}\times t)+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/7be48.png)
![\frac{1}{[A]_{0}}=0.707M^{-1}s^{-1}\times t](/tpl/images/0385/0648/fecfa.png)