Chemistry, 21.11.2019 21:31, shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1
Do you know the correct answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions in other subjects:

Social Studies, 19.08.2019 16:10





Chemistry, 19.08.2019 16:10




Mathematics, 19.08.2019 16:10


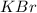 :
:
 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol
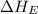 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol