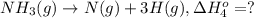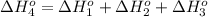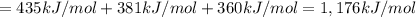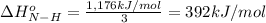Chemistry, 21.11.2019 20:31, nulledcracker12
From the following data, calculate the average bond enthalpy for the noh bond: nh3(g) ¡nh2(g) 1 h(g) ¢h° 5 435 kj/mol nh2(g) ¡nh(g) 1 h(g) ¢h° 5 381 kj/mol nh(g) ¡n(g) 1 h(g) ¢h° 5 360 kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2
Do you know the correct answer?
From the following data, calculate the average bond enthalpy for the noh bond: nh3(g) ¡nh2(g) 1 h(g...
Questions in other subjects:




Computers and Technology, 10.12.2020 20:20



Mathematics, 10.12.2020 20:20

Mathematics, 10.12.2020 20:20

English, 10.12.2020 20:20


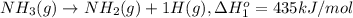 ..[1]
..[1]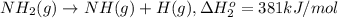 ..[2]
..[2]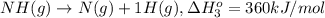 ..[3]
..[3]