Chemistry, 21.11.2019 20:31, rileyeddins1010
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to inflate airbags: 2 nan3 (s) 2 na (s) + 3 n2 (g) how many grams of n2 are produced from 6.00 g of nan

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1
Do you know the correct answer?
The rapid decomposition of sodium azide, nan3, to its elements is one of the reactions used to infla...
Questions in other subjects:



Mathematics, 05.10.2019 10:00

Mathematics, 05.10.2019 10:00



History, 05.10.2019 10:00




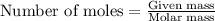 .....(1)
.....(1)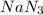 = 6.00 g
= 6.00 g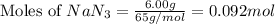
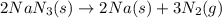
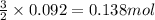 of nitrogen gas
of nitrogen gas