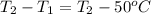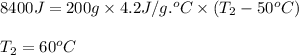Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
Do you know the correct answer?
Ahot iron ball is dropped into a 200. g sample of water initially at 50°c. if 8.4 kj of heat is tran...
Questions in other subjects:

History, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10


Mathematics, 27.05.2021 19:10


Chemistry, 27.05.2021 19:10

SAT, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10

English, 27.05.2021 19:10

Mathematics, 27.05.2021 19:10


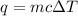
 = change in temperature =
= change in temperature =