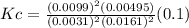Chemistry, 21.11.2019 09:31, thatonestudent2271
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equilibrium the concentration of no in the vessel is 0.161m. at equilibrium the vessel also contains n2, h2o, and h2. what is the value of the equilibrium constant for kc for the following reaction?
2h2 (g) + 2no(g) < —> 2h2o (g) + n2 (g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, lifeofabe214
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
Do you know the correct answer?
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equ...
Questions in other subjects:

Mathematics, 26.06.2019 08:30

English, 26.06.2019 08:30



Mathematics, 26.06.2019 08:30

Mathematics, 26.06.2019 08:30



History, 26.06.2019 08:30

English, 26.06.2019 08:30


 ml
ml moles
moles moles
moles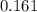 M
M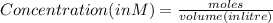
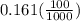
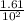 moles
moles
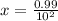
![Kc=\frac{[H2O]^2[N2]}{[H2]^2[NO]^2} (volume of vesselin litre)](/tpl/images/0384/4648/de870.png)