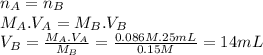Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m koh would need to be added to 25 ml of 0.086 m lactic acid to reach the equivalence point? (keep 2 significant figures) ml (b) at the equivalence point, would the aqueous solution be acidic, basic, or neutral? explain why at the equivalence point, the solution will be .. at this stage, all of the lactic acid in the solution will have reacted with the koh added, producing lactate lons (c3h503") and potassium ions (*) in the solution. the potassium ions will not affect the ph, but the lactate ions will make the solution -

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
Do you know the correct answer?
Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m ko...
Questions in other subjects:

Mathematics, 22.07.2021 15:40

Mathematics, 22.07.2021 15:40


Mathematics, 22.07.2021 15:40

History, 22.07.2021 15:40



Mathematics, 22.07.2021 15:40