

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 05:10, citlalli30
Name a brittle metal , which is used to galvanize iron
Answers: 1
Do you know the correct answer?
Heat is transferred at a rate of 2 kw from a hot reservoir at 775 k to a cold reservoir at 300 k. ca...
Questions in other subjects:

Mathematics, 08.10.2020 07:01

Mathematics, 08.10.2020 07:01


Mathematics, 08.10.2020 07:01

English, 08.10.2020 07:01


Mathematics, 08.10.2020 07:01

Mathematics, 08.10.2020 07:01


Mathematics, 08.10.2020 07:01


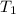 = 775 K
= 775 K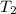 = 300 K
= 300 K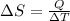
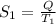
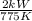
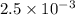 kW/K
kW/K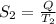
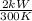
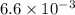 kW/K
kW/K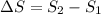
 kW/K
kW/K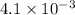 kW/K
kW/K



