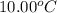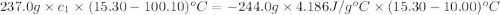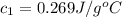A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup containing 244.0 g of water at 10.00 °c. if the final temperature of the water and metal in the cup is 15.30 °c, then what is the specific heat of molybdenum? (specific heat of water = 4.186 j/g-°c do not add the unit in the answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2


Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
Do you know the correct answer?
A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup co...
Questions in other subjects:






Biology, 23.06.2019 07:00



Chemistry, 23.06.2019 07:00


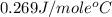
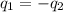
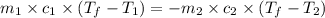
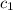 = specific heat of molybdenum metal = ?
= specific heat of molybdenum metal = ?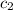 = specific heat of water =
= specific heat of water = 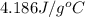
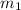 = mass of molybdenum metal = 237.0 g
= mass of molybdenum metal = 237.0 g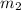 = mass of water = 244.0 g
= mass of water = 244.0 g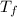 = final temperature of water and metal =
= final temperature of water and metal = 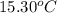
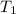 = initial temperature of molybdenum metal =
= initial temperature of molybdenum metal = 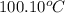
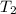 = initial temperature of water =
= initial temperature of water =