Chemistry, 21.11.2019 06:31, mraymundo025p0gpw9
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the energy of the universe is constant; the entropy of the universe increases.3) if a process increases the randomness of the particles of a system, the entropy of the system increases.4) all systems become more disordered spontaneously.5) all spontaneous processes release heat.6) both δ ssys and δ ssurr must equal zero at equilibrium.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
Do you know the correct answer?
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the...
Questions in other subjects:


Geography, 16.09.2019 19:30


Geography, 16.09.2019 19:30







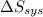 and
and 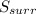 must equal zero at equilibrium.
must equal zero at equilibrium.