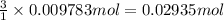The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with hydrogen: wo3(s)+3h2(g)→w(s)+3h2o(g)
part a how many grams of tungsten trioxide must you start with to prepare 1.80 g of tungsten? (for wo3, mw = 231.8 amu.)
part b how many grams of hydrogen must you start with to prepare 1.80 g of tungsten?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2



Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
Do you know the correct answer?
The tungsten metal used for filaments in light bulbs is made by reaction of tungsten trioxide with h...
Questions in other subjects:



History, 09.07.2019 01:00

Chemistry, 09.07.2019 01:00


Health, 09.07.2019 01:00

Business, 09.07.2019 01:00

History, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00


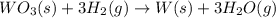
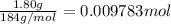
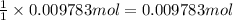 of tungsten trioxide
of tungsten trioxide