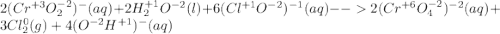Consider the following balanced redox reaction (do not include state of matter in your answers): 2cro2−(aq) + 2h2o(l) + 6clo−(aq) →2cro42−(aq) + 3cl2(g) + 4oh−(aq)(a) which species is being oxidized? (b) which species is being reduced? (c) which species is the oxidizing agent? (d) which species is the reducing agent? (e) from which species to which does electron transfer occur?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
Do you know the correct answer?
Consider the following balanced redox reaction (do not include state of matter in your answers): 2cr...
Questions in other subjects:



Mathematics, 23.04.2021 19:10


Mathematics, 23.04.2021 19:10

Mathematics, 23.04.2021 19:10

Mathematics, 23.04.2021 19:10

Mathematics, 23.04.2021 19:10

Computers and Technology, 23.04.2021 19:10

History, 23.04.2021 19:10