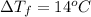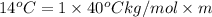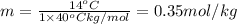Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
Do you know the correct answer?
Calculate the molality of isoborneol in the product if, a) the melting point of pure camphor is 179°...
Questions in other subjects:

Mathematics, 26.09.2021 02:30


English, 26.09.2021 02:30


Mathematics, 26.09.2021 02:30

Biology, 26.09.2021 02:30

English, 26.09.2021 02:30

Spanish, 26.09.2021 02:30

Mathematics, 26.09.2021 02:30

Biology, 26.09.2021 02:30


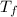 = 165°C
= 165°C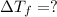
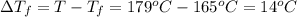
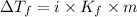
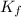 = The freezing point depression constant
= The freezing point depression constant