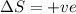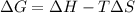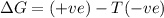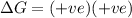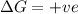Chemistry, 20.11.2019 23:31, annjetero2oy23ay
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably unfavorable; unfavorable; nonspontaneous unfavorable; favorable; spontaneous favorable; unfavorable; spontaneous favorable; unfavorable; nonspontaneous unfavorable; unfavorable; spontaneous

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
Do you know the correct answer?
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction i...
Questions in other subjects:

Mathematics, 10.12.2019 11:31

Mathematics, 10.12.2019 11:31

Mathematics, 10.12.2019 11:31





Mathematics, 10.12.2019 11:31


Mathematics, 10.12.2019 11:31


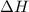 for Endothermic reaction is positive and
for Endothermic reaction is positive and 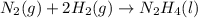
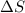 is negative as the randomness decreases when gases convert into liquid.
is negative as the randomness decreases when gases convert into liquid. 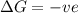 and favourable conditions are
and favourable conditions are 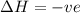 and
and