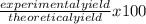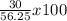Chemistry, 20.11.2019 23:31, jacobs5919
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + cl2 → c6h5cl + hcl , what is the percent yield of c6h5cl? 1. 50.0% 2. 53.4% 3. 69.4% 4. 13.2% 5. 76.9%

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
Do you know the correct answer?
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + c...
Questions in other subjects:

Computers and Technology, 06.11.2020 23:40

Physics, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40


Mathematics, 06.11.2020 23:40

Chemistry, 06.11.2020 23:40

Mathematics, 06.11.2020 23:40


Mathematics, 06.11.2020 23:40

History, 06.11.2020 23:40