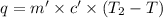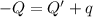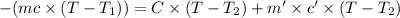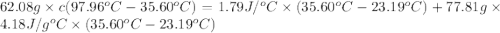In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 62.08 grams of magnesium to 97.96 °c and then drops it into a cup containing 77.81 grams of water at 23.19 °c. she measures the final temperature to be 35.60 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.79 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of magnesium.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
Do you know the correct answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions in other subjects:

Physics, 31.08.2019 22:00

Mathematics, 31.08.2019 22:00

History, 31.08.2019 22:00



History, 31.08.2019 22:00



Mathematics, 31.08.2019 22:00

History, 31.08.2019 22:00


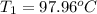
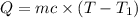
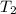 = 23.19°C
= 23.19°C