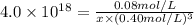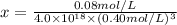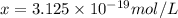Chemistry, 20.11.2019 21:31, marlesly87
Ethylenediamine (en) forms an octahedral complex with ni2+(aq) with the formula [ni(en)3]2+. ni2+(aq) + 3 en ⇌ [ni(en)3]2+(aq) kf = 4.0 x 1018 if there are 0.16 mol [ni(en)3]2+ and 0.80 mol ethylenediamine at equilibrium in a 2-l solution, what is the concentration of ni2+(aq) in the solution?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
Ethylenediamine (en) forms an octahedral complex with ni2+(aq) with the formula [ni(en)3]2+. ni2+(aq...
Questions in other subjects:


History, 01.08.2019 02:40

Social Studies, 01.08.2019 02:40

Chemistry, 01.08.2019 02:40







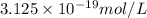 is the concentration of
is the concentration of 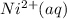 in the solution.
in the solution.![Ni^{2+}(aq) + 3 en\rightleftharpoons [Ni(en)_3]^{2+}(aq)](/tpl/images/0383/3334/4ce74.png)
![[Ni^{2+}]=x](/tpl/images/0383/3334/f3c8f.png)
![[[Ni(en)_3]^{2+}]=\frac{0.16 mol}{2 L}=0.08 mol/L](/tpl/images/0383/3334/80efd.png)
![[en]=\frac{0.80 mol}{2 L}=0.40 mol/L](/tpl/images/0383/3334/3eb02.png)
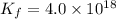
![K_f=\frac{[[Ni(en)_3]^{2+}]}{[Ni^{2+}][en]^3}](/tpl/images/0383/3334/2ab0f.png)