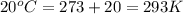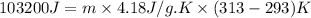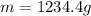Chemistry, 20.11.2019 20:31, bougiehairstudios
For many years drinking water has been cooled in hot climates by evaporating it from the surface of canvas bags or porous clay pots. how many grams of water can be cooled from 40 ∘c to 20 ∘c by the evaporation of 43 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj/g. the specific heat of water is 4.18 j/g⋅k.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1



Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
Do you know the correct answer?
For many years drinking water has been cooled in hot climates by evaporating it from the surface of...
Questions in other subjects:

Biology, 26.03.2021 23:00


Mathematics, 26.03.2021 23:00





Health, 26.03.2021 23:00


Mathematics, 26.03.2021 23:00


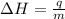
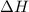 = enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g
= enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g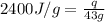
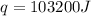
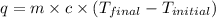
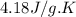
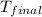 = final temperature =
= final temperature = 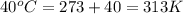
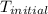 = initial temperature =
= initial temperature =