

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 22:30, vhife4901
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
Do you know the correct answer?
Gthe following reaction is the first step in the production of nitric acid from ammonia. 4nh3(g) 5o2...
Questions in other subjects:

Arts, 08.12.2020 19:00

History, 08.12.2020 19:00

Mathematics, 08.12.2020 19:00


Mathematics, 08.12.2020 19:00


Social Studies, 08.12.2020 19:00


Mathematics, 08.12.2020 19:00



![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0383/2489/18a63.png)

![\Delta H_{rxn}=[(4\times \Delta H_f_{(NO(g))})+(6\times \Delta H_f_{(H_2O(g))})]-[(4\times \Delta H_f_{(NH_3(g))})+(5\times \Delta H_f_{(O_2)})]](/tpl/images/0383/2489/02e37.png)
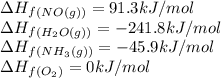
![\Delta H_{rxn}=[(4\times (91.3))+(6\times (-241.8))]-[(4\times (-45.9))+(5\times (0))]\\\\\Delta H_{rxn}=-902kJ](/tpl/images/0383/2489/6f6da.png)




