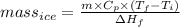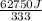Chemistry, 20.11.2019 20:31, jdsfdujfi1598
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitcher. if the tea is initially at 30.0°c and the ice cubes are initially at 0.0°c, how many grams of ice will still be present when the contents of the pitcher reach a final temperature? the tea is mostly water, so assume that it has the same density (1.0 g/ml), molar mass, heat capacity (75.3 j/k/mol), and heat of fusion (6.01 kj/mol) as pure water. the heat capacity of ice is 37.7 j/k/mol.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
Do you know the correct answer?
You make some iced tea by dropping 325 grams of ice into 500.0 ml of warm tea in an insulated pitche...
Questions in other subjects:


Chemistry, 01.09.2021 14:00



Mathematics, 01.09.2021 14:00



Chemistry, 01.09.2021 14:00


Chemistry, 01.09.2021 14:00


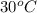 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K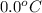 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K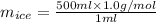
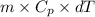
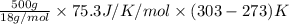
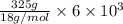
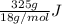 heat but we have 40774.95 J.
heat but we have 40774.95 J.