
Chemistry, 20.11.2019 07:31, kittycat92
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid whose vapor pressure is the same as that of pure water at 20.0°c (18 torr). the volume of the mixture is 1.7 l, and its total pressure is 0.610 atm. determine the partial pressure of the hydrogen gas in this mixture.
a. 446 torr
b. 788 torr
c. 758 torr
d. 464 torr
e. 482 torr

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
Do you know the correct answer?
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid who...
Questions in other subjects:

SAT, 23.12.2021 19:00







SAT, 23.12.2021 19:10


Spanish, 23.12.2021 19:10


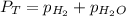
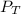 = total partial pressure = 0.610 atm = 463.6 torr
= total partial pressure = 0.610 atm = 463.6 torr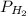 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?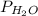 = partial pressure of water vapor = 18 torr
= partial pressure of water vapor = 18 torr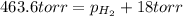
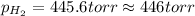
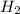 gas is, 446 torr
gas is, 446 torr



