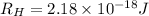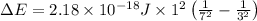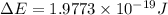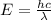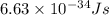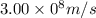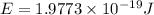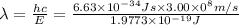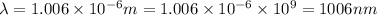Given that rh= 2.18 x 10⁻¹⁸j, 1 nm = 1 x 10⁻⁹m, h = 6.63 x 10⁻³⁴j·s, and c = 3.00 x 10⁸m/s:
c...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
Questions in other subjects:






Mathematics, 29.11.2020 19:30




History, 29.11.2020 19:30


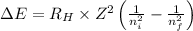
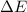 = Energy difference
= Energy difference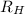 = Rydberg's Constant
= Rydberg's Constant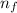 = Final energy level
= Final energy level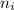 = Initial energy level
= Initial energy level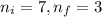 , Z = 1
, Z = 1