

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2
Do you know the correct answer?
The rate of disappearance of hbr in the gas phase reaction 2hbr(g) → h2 (g) i2 (g) is 0.190 m s-1 at...
Questions in other subjects:

Physics, 05.12.2019 15:31

Mathematics, 05.12.2019 15:31

Mathematics, 05.12.2019 15:31


Mathematics, 05.12.2019 15:31

English, 05.12.2019 15:31





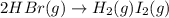
![-\frac{d[HBr]}{dt}=0.190 M/s](/tpl/images/0382/0303/cb882.png)
![R=-\frac{1}{2}\frac{d[HBr]}{dt}=\frac{1}{1}\frac{d[H_2]}{dt}](/tpl/images/0382/0303/fdda3.png)
![R=-\frac{1}{2}\frac{d[HBr]}{dt}=\frac{1}{2}\times 0.190 M/s=0.095 M/s](/tpl/images/0382/0303/382d0.png)
![\frac{d[H_2]}{dt}=R =0.095 M/s](/tpl/images/0382/0303/0c033.png)




