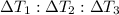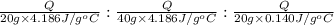Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity is 0.140 joules/gram degree celsius.
water and mercury are put into three identical bowls:
bowl a contains 20 grams of water.
bowl b contains 40 grams of water.
bowl c contains 20 grams of mercury.
the bowls start at the same temperature, and then the same amount of heat is added to each bowl. order the bowls from coolest to warmest, based on their final temperatures.
bowl a

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 23.06.2019 10:10, nancysue1975
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

Chemistry, 23.06.2019 16:20, kristalmakhija
Which of the following subject areas contains questions that can be answered by science? alchemy ethics forensics politics
Answers: 1
Do you know the correct answer?
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity...
Questions in other subjects:

Computers and Technology, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Chemistry, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10





Mathematics, 18.03.2021 01:10


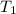
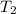
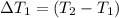
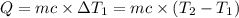
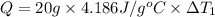
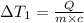
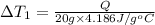 ..[1]
..[1]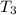
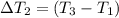
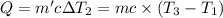
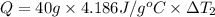
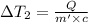
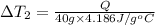 ..[2]
..[2]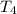
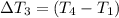
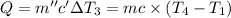
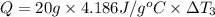
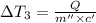
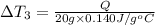 ..[3]
..[3]