

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2



Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2
Do you know the correct answer?
Consider the complete combustion of glucose (c6h12o6) with o2 and calculate the moles of co2 produce...
Questions in other subjects:

Mathematics, 12.11.2020 23:30


Mathematics, 12.11.2020 23:30


History, 12.11.2020 23:30


English, 12.11.2020 23:30

Mathematics, 12.11.2020 23:30



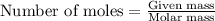
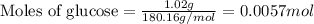
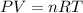
![37^oC=[37+273]K=310K](/tpl/images/0381/5720/20b22.png)
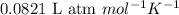
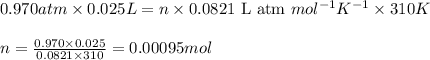
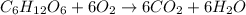
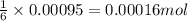 of glucose
of glucose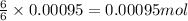 of carbon dioxide
of carbon dioxide




