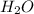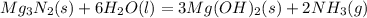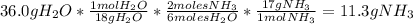Chemistry, 19.11.2019 18:31, nadiarose6345
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced? when 36.0 g of h2o react, how many grams of nh3 are produced? 34.0 g 10.0 g 5.67 g 11.3 g 102 g

Answers: 2
Similar questions


Chemistry, 20.09.2019 01:30, jlchandl
Answers: 1

Chemistry, 12.10.2019 07:30, DWilson1234
Answers: 1

Chemistry, 14.11.2019 07:31, theblondeone2
Answers: 2
Do you know the correct answer?
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced?...
Questions in other subjects:



Mathematics, 28.06.2021 15:10







Mathematics, 28.06.2021 15:10


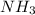 are produced from 36.0 g of
are produced from 36.0 g of